Why would electrical engineers or electricians be interested in conductors and insulators? Because different types of materials are used for manufacturing electrical products and equipment. Any material in real life can have different electrical properties and characteristics. That can be used appropriately for better utilization in the electrical engineering field. But electrically, a material can be classified as either a conductor, a semiconductor, or an insulator.
The property that makes a material an insulator, conductor, or semiconductor is electrical resistivity. Electrical resistivity is the measure of how difficult a material is for a current. Which depends upon the availability of free electrons or ions. The more free electrons/ions available in a body, the more it will be a conductor, and fewer electrons/ions mean an insulator.
Electrical resistivity is the resistance of a one-meter cube volume wire; its SI unit is Ohm-Meter (Ω-m) and is represented with the Greek letter ρ (ROH). Electrical conductivity is the reciprocal of resistivity. And it means to measure the ability to conduct an electric current. The SI unit for electrical conductivity is Siemens/meter (S/m). The resistivity/conductivity varies from material to material.
What are Conductors, semiconductors, and insulators?
Let’s have a close look at them.
What is a Conductor?
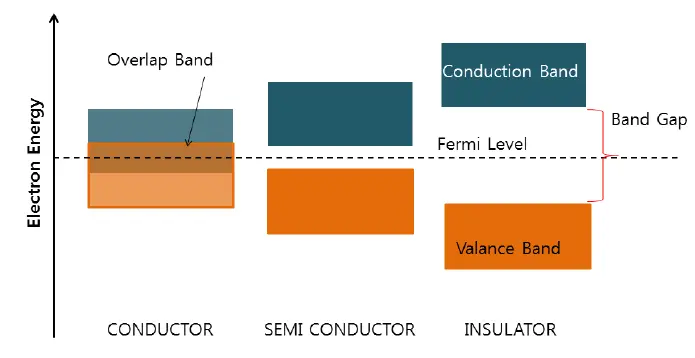
Conductors are types of materials that pass electric current through them because of the availability of free electrons. An electrical conductor has low resistivity and high conductivity. The resistivity of a conductor is from zero to 10-4 Ohm-meter. The conductor has plenty of free electrons in it, and a small potential can cause the flow of electrons.
One good example of a conductor is metal. There are very few valence electrons in the outermost shell of each atom, and they are loosely attracted to the nucleus. Very little energy can be extracted from the atom’s orbit and make it free electrons.
Electrical wire is made of conductors that carry current from the source to the load. The best electrical conductors are copper, silver, gold, etc.
What is an Insulator?
Insulators are a type of electrical material that possesses high resistivity. It has a resistivity of more than 104 Ohm-meters, or inversely, low conductivity. There are almost no free electrons available for carrying current in insulators. A large voltage in the insulator may not cause a current because of the unavailability of free electrons. The insulator may have a very small current called a leakage current.
Most non-metals are insulators, where bonds have a desire to catch more electrons. Therefore, a significant amount of external energy may not be able to extract an electron-free. In an ionic material, all the ions are arranged together such that there are no carriers available for the current to carry.
Insulators are used to support the conductor, avoid electric shock, and prevent current from flowing through all those paths where no current is intended. The best insulators are ceramics, plastic, wood, etc.
What is a Semiconductor?
The semiconductor has a resistivity between 10-4 and 104 ohm-meters. At normal temperatures, the resistivity of a semiconductor is comparatively high. But as the temperature increases, the resistivity decreases. A semiconductor mostly has four electrons in its valence shell and belongs to the 3rd and 4th rows of the periodic table.
The number of free electrons in a semiconductor is very small at ambient temperature. Where external energy, i.e. increase in temperature, can increase the number of free electrons. A semiconductor is the basis of modern electronics. The best examples of semiconductors are Germanium, Silicon, Gallium Arsenide, etc.
Conclusion
In electrical and electronics engineering, the material can be divided into three categories. The conductor, semiconductor, and insulator are based on the number of free electrons at the atomic level.